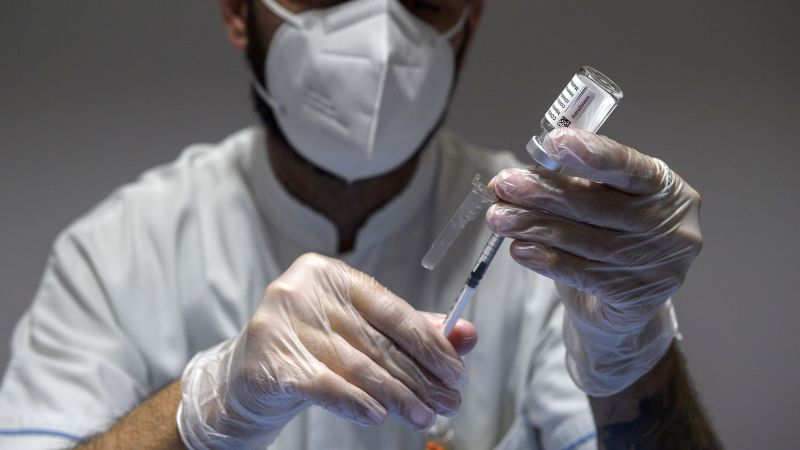
AstraZeneca Withdraws Marketing Authorizations for Covid-19 Vaccine as Demand Declines
In June 2021, a healthcare worker prepares doses of the AstraZeneca Covid-19 vaccine at a vaccine hub in Rome. Since its launch in January 4, 2021, Vaxzevria, the coronavirus vaccine developed with the University of Oxford and distributed by AstraZeneca, has been administered to over 3 billion people globally. However, despite its success, the vaccine has not generated any revenue for AstraZeneca since April 2023.
The decline in demand for Vaxzevria is attributed to the availability of numerous updated vaccines on the market. Consequently, AstraZeneca has decided to withdraw marketing authorizations for Vaxzevria within Europe. The European Medicines Agency (EMA) has also announced that it will withdraw Vaxzevria from the market in all European Union countries.
AstraZeneca expressed pride in Vaxzevria’s role in combating the global pandemic and its recognition by governments worldwide. Despite this decision, they will work with regulators in other countries to align on a clear path forward and withdraw marketing authorizations for the vaccine where no future commercial demand is expected. As this is an evolving story, updates will be provided as they become available.

